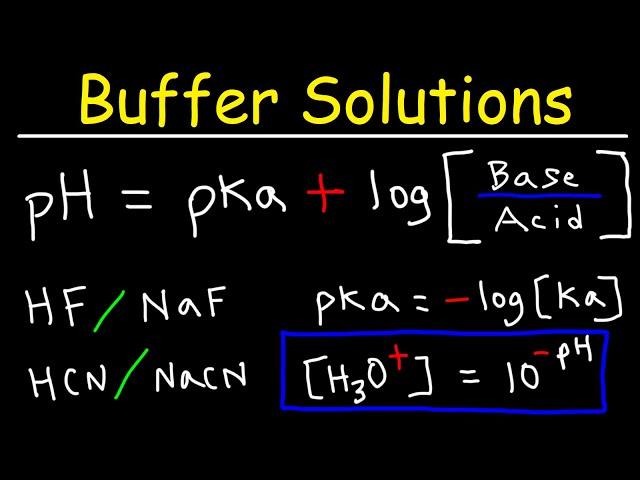
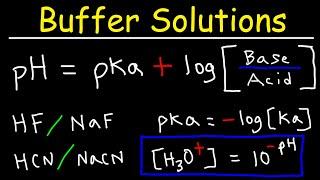
Комментарии:

How can i identify weak acid?😢
Ответить
God bless you so much
Ответить
I think whats especially confusing is the Ka value is supposed to describe the disassociation of the ions, which is supposed to dictate the concentration of reactants/products, so its weird to understand how the concentrations of Acid vs Conj base can be equal
Ответить
ها طلاب السادس 😂😂
Ответить
Sir love the work, all your videos have been helpful in all subjects. You have my sincere encouragement
Ответить
Bro is much better than my chemistry teacher ☠️👍
Ответить
Anyone doing chemistry exam tomorrow gather here.😍
Ответить
Awesome! So clear.
Ответить
Mr. The Organic Chemistry Tutor sir, do you have a video about Complexometric Titrations? Your videos are always easy to understand and frankly speaking I cannot find other videos that helped me in this topic😢😢
Ответить
I love you organic chemistry teacher.
Ответить
Thank you 😊 school made easy ❤
Ответить
Anyone see question 5
Ответить
In this video we're gonna talk about....😊
Ответить
Your videos are perfect
Ответить
Y didn't you consider 750 because da number of moles must be in 1000
Ответить
University takes money for no reason
Ответить
My professor has a thick french accent so everything was harder to understand since chemistry lectures already sound french to me😢
Ответить
Thank you I’m taking my ap exam today and I now understand these stupid buffers for the first time this year
Ответить
Thank you!!
Ответить
Thank you ❤❤❤🥺
Ответить
Thank you v❤
Ответить
❤❤❤❤❤❤
Ответить
Please help how to calculate molar ratio
Ответить
School fucking sucks
Ответить
Very cool! Thank you organic chemistry tutor !
Ответить
You're the best sir❤❤❤❤
Ответить
You’re so good at your job
Ответить
so the ratio of base to acid in the logarithm is it between concentration or amount
Ответить
so we can use this equation with molarity and moles?
Ответить
So for buffer problems we dont need to use ice tables?
Ответить
Barr's Chem 20B gang rise up
Ответить
is it conjugated weak base or conjugated strong base ??
According to Bronsted–Lowry acid–base theory, the stronger of a pair of acids must form the weaker of a pair of conjugate bases. Therefore, the conjugate base of a strong acid is a weak base.( from google)

Sooooo welll explained
Ответить
So your last step in calculating log of b/a is incorrect as your adding to pH you should strictly implicate that you subtract log of b/a which will decrease pH. That’s how at least I got 2.977
Ответить
Thanks
Ответить
What to do if HA is the one u need to find in the equation
Ответить
I have a question. On problem 3 can you not divide by 750 ml instead of the molar mass to get the moles?
Ответить
Having an exam tomorrow... I'm sure it'd help me ace any buffer related question
Ответить
You are amazing
Ответить
i love u
Ответить
I LOVE YOU
Ответить
This channel is still saving me in college ❤ thank you for those videos
Ответить
You are a good man ,sir I don't remember how much you help me to be good in my academic performance
Ответить
I wish there was a "The Organic Chemistry Tutor" version for Biology related stuffs 😔.
I don't have issues with my Physics and Chemistry because of this man, but for my biology 😭😭😭

because of you I will probably have a good grade tomorrow. You're videos are the best in the game, thank you.
Ответить
If you have 15g of HF in 1 liter of solution that would be 0.75M, but you have 15g In 750ml, which means you a 1M solution of HF?
Ответить
Thank you
Ответить