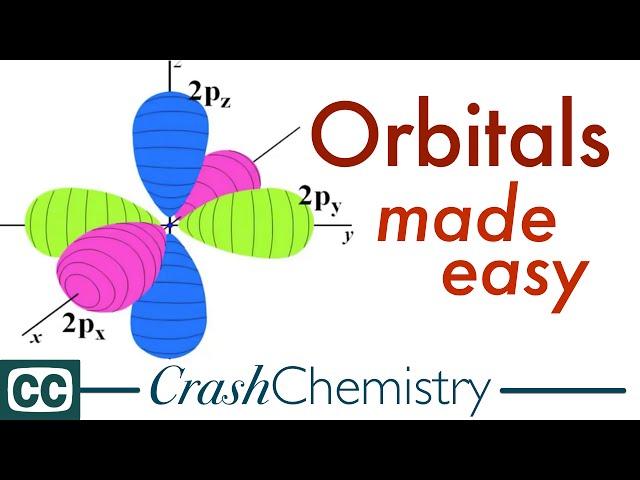
Orbitals, the Basics: Atomic Orbital Tutorial — probability, shapes, energy |Crash Chemistry Academy
Комментарии:

Great lecture! But now, what are pi and sigma bonds?
Ответить
reviewing for my MCAT rn very helpful!!
Ответить
love u my man keep on clashing
Ответить
this helped so much in understanding with orbitals, the only video that really helped me
Ответить
This is exactly what i needed
Ответить
This was a really nice video. You explained everything so simply. Thank you.
Ответить
This is amazing, thanks
Ответить
THE BEST VIDEO EVER, thank you!!!!!
Ответить
has anyone tried to extrapolate out these patterns into what shapes must be forming cross-sections that are visible like that?
Ответить
The electron can be found where it is, but the speed ha to be syncronised with the first orbit. It needs the wave of color blu. Very simply.
Ответить
Was going through more than 10 videos on YT and still couldn't figure out what an orbital is. This video definitely helped me understand the difference between the orbital and the orbit.
Ответить
Do you see how irrational this theory is? That's why it is often presented in a mixed way as electrons orbit along strange trajectory withing an orbital represented as an field of a given shape. But when we can observe individual atoms at play, we see quite stable behavior. Look around you. There is no coincidence what is made of what and why. Also it is quite persistent in many cases like damn brick in a wall. That probability approach is ridiculous. The shape of the field matters and says everything. This is the inner part, a nucleus that decides the shape of the outer electric filed. As it is afield than an electron as concept is useless and makes the whole confusion. The field changes by equal parts, so the shape of the field. The shape matters. Look at methane with graphical representation of orbitals for example. It is all there, the whole mystery of chemistry and physics in one spot without guessing where the electron is. Charge is charge and must be conserved.
Ответить
thank you!!!
Ответить
Wonderfull class, all of this is to get 90% chance of finding the electron, and let's say i found one. What i supose to do with it? Bringing home e pet my electron?
Joke aside there is people that say the location of the electron is not so important as to find out the momentum witch they have, i don't know if that's true tough...

Bro, you have no idea of how much I've helped me! Thank you very much!
Ответить
Thank you!!! I've watched 3 videos already on orbitals and was completely lost. Breaking it down visually was super helpful.
Ответить
Why can an electron be outside it's orbital by a probability of 10%?
Ответить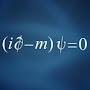
Why is "p" orbital not a sphere anymore?
Ответить
Fantastic visuals! Thank you🙏🏻
Ответить
Only video that fully explains this
Ответить
This was absolutely amazing!!! Been trying to learn this for the past week and your video finally made it click! Legend!
Ответить
cool
Ответить
This video was really helpful. Can't thank you enough for explaining this thing so nicely.
Ответить
Really hellpful! Thank you!
Ответить
Thank you so much!
Ответить
Awesome Video😎👍
Ответить
Thank you so very much
Ответить
this video is superb. I ve been learning with tutors and reading tru textbooks, I still get really confused. you are amazing
Ответить
Thank you so much you explained everything so clearly.
Ответить
"Everything, Everywhere, All at Once".
Ответить
I've been trying to understand this topic for over 2 years. (I actually learned it 2 years ago, didn't understand it, and now have to learn it again) and I FINALLY UNDERSTAND. Thanks for being everything that my chemistry teacher should have been!!!!!
Ответить
exactly what i needed thanks. IDK why it so hard for a prof to teach like this.
Ответить
Thank you for walking through this. I finally understand the topic I never could never grasp years ago either because textbooks don’t break it down this way or professors assume we already know
Ответить
11 yr ago 👍
Ответить
The best explanation of orbitals. the sentence ( the shape is the probability of the position of the electrons, not the path) explained confusion for two semesters trying to grasp what these shapes are. Thank you so much!
Ответить
i wish this guy made more tutorials. I hope he isn't six feet under.
Ответить
Nice crash course of electron orbitles !
Ответить
This vid is very nice
Ответить
Why all the above is important? 😮
To understand the implementation of the Legendre Polynomial in conjunction with the quantum number (n)², giving us 7²=49 Orbitals, each with 3 Electrons = 98 Electrons total in Californium, compared to only 94 Electrons, that is 4 Electrons less in 5th Engery Level, or Shell, in Plutonium with the same number of shells and thus the same number of expected Orbitals.😵💫
Conclusion: The rudementary method of calculating the number of electrons in an atom by knowing the number of orbitals does not always work out. 😮💨

As a physical learner this video really help thank you
Ответить
very good video I love it
Ответить
ASMR????
Ответить
Are you drinking a shake?
Ответить
THANK U SO MUCH FOR THE VIDEO! IM ABOUT TO HAVE MY FIRST UNI LEVEL CHEM FINAL TOMORROW AND IT'S SO NICE TO SEE THIS VIDEO, I FINALLY UNDERSTAND THINGS!!!
Ответить
Omg thx I did not understand my teachers way of explaining it was very confusing thx for this lecture it helped it
Ответить
merci
Ответить
hi you are a amazing
Ответить








![Hitler privat: Der Künstler [Teil 1] | SPIEGEL TV Hitler privat: Der Künstler [Teil 1] | SPIEGEL TV](https://ruvideo.cc/img/upload/ZzVpYlpBZVJ2eTE.jpg)