Talk: On the generation of theta rhythms and theta-gamma phase amplitude coupling.
Speaker: Alexandra Chatzikalymniou, University of Toronto (grid.17063.33)
Title: On the generation of theta rhythms and theta-gamma phase amplitude coupling.
Emcee: Roozbeh Farhoodi
Backend host: Pavithra Rajeswaran
Details: https://neuromatch.io/abstract?submission_id=recVvbU5XN2YTDaoj
Paper link: https://www.biorxiv.org/content/10.1101/2020.07.28.225557v1
Twitter: https://twitter.com/Alex_Pierri_C
Presented during Neuromatch Conference 3.0, Oct 26-30, 2020.
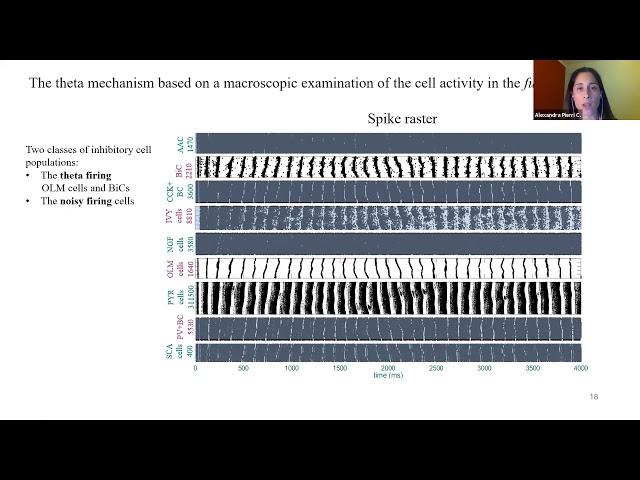
Summary: In the hippocampus, the 3-12 Hz local field potential (LFP) theta oscillation is a rhythm strongly correlated with spatial navigation, episodic memory and rapid-eye-movement (REM) sleep. As such, understanding the origin of this rhythm could shed light on the emergence of such cognitive phenomena. However, how the large variety of inhibitory and excitatory cell types form a theta-generating network remains unknown. We use a previously published full CA1 hippocampus model of unprecedented biological detail to understand the generation mechanism of theta rhythms. The model contains eight morphologically detailed interneuron types and pyramidal cells and is constructed based on experimentally derived connectivity. We find that a 400 μm thick, isolated segment (slice) of this hippocampus model, represents the minimum network that can generate theta rhythms. Guided explorations of this computationally tractable model reveal that theta rhythms are initiated by the pyramidal cells but are regularized by inhibitory inputs from parvalbumin positive fast-firing cells, supporting an “inhibition-based tuning” mechanism. We find a strong correlation between the net input current to the pyramidal cells and the resulting theta frequency, inferring that the intrinsic pyramidal cell properties are theta frequency controllers. We next investigate the control of external input drives to the theta rhythms of the segment model, including the recently discovered inputs from the subiculum. Finally, we leverage our insights to explain the termination mechanism of theta bursts and the theta/gamma phase-amplitude coupling in the original full CA1 model. As modern pharmacological approaches focus on cellular and subcellular targets, our models can be used to identify cellular-specific pathways underlying changes in LFP theta/gamma rhythms. In turn, this opens up avenues for clinical translation.
Title: On the generation of theta rhythms and theta-gamma phase amplitude coupling.
Emcee: Roozbeh Farhoodi
Backend host: Pavithra Rajeswaran
Details: https://neuromatch.io/abstract?submission_id=recVvbU5XN2YTDaoj
Paper link: https://www.biorxiv.org/content/10.1101/2020.07.28.225557v1
Twitter: https://twitter.com/Alex_Pierri_C
Presented during Neuromatch Conference 3.0, Oct 26-30, 2020.
Summary: In the hippocampus, the 3-12 Hz local field potential (LFP) theta oscillation is a rhythm strongly correlated with spatial navigation, episodic memory and rapid-eye-movement (REM) sleep. As such, understanding the origin of this rhythm could shed light on the emergence of such cognitive phenomena. However, how the large variety of inhibitory and excitatory cell types form a theta-generating network remains unknown. We use a previously published full CA1 hippocampus model of unprecedented biological detail to understand the generation mechanism of theta rhythms. The model contains eight morphologically detailed interneuron types and pyramidal cells and is constructed based on experimentally derived connectivity. We find that a 400 μm thick, isolated segment (slice) of this hippocampus model, represents the minimum network that can generate theta rhythms. Guided explorations of this computationally tractable model reveal that theta rhythms are initiated by the pyramidal cells but are regularized by inhibitory inputs from parvalbumin positive fast-firing cells, supporting an “inhibition-based tuning” mechanism. We find a strong correlation between the net input current to the pyramidal cells and the resulting theta frequency, inferring that the intrinsic pyramidal cell properties are theta frequency controllers. We next investigate the control of external input drives to the theta rhythms of the segment model, including the recently discovered inputs from the subiculum. Finally, we leverage our insights to explain the termination mechanism of theta bursts and the theta/gamma phase-amplitude coupling in the original full CA1 model. As modern pharmacological approaches focus on cellular and subcellular targets, our models can be used to identify cellular-specific pathways underlying changes in LFP theta/gamma rhythms. In turn, this opens up avenues for clinical translation.
Комментарии:
Talk: On the generation of theta rhythms and theta-gamma phase amplitude coupling.
Neuromatch Conference
NEET 2024 | Counselling Details | IMPORTANT DOCUMENTS | 10/06/2024 | 7:30 PM Owards
Brilliant Pala - NEET
ShockWave on 3DO Part 1 of 2
KillerGameRants KGR
«Кария» 1 серия / «Қария» 1-ші бөлім
Khabar TV
Awazê Çiya - Rabe
Nefel Production