Комментарии:

WOW!!!!!!
Ответить
Amazing!
Ответить
I FOUND YOU ATLAST WAVE FUNCTION!!!... this should've been in physics... but its begining in chem, then goes to phy. ... cooool!
Ответить
nice!
Ответить
This is Awesome!
Ответить
Fundamentally illuminating!
Ответить
smoke weed every day
Ответить
Nerdologia
Ответить
Helped me a lot,thank u
Ответить
Thank you very much, this was incredibly helpful!
Ответить
helpful!
Ответить
Could someone please help me identify the orbitals....I lost track after the second one when the flowery stuff kicked in.Would be a great help. :)
Ответить
this is wroooooong
Ответить
tell me thats not how our galaxy works
Ответить
excellent for visualization
Ответить
Excellent! Thank you
Ответить
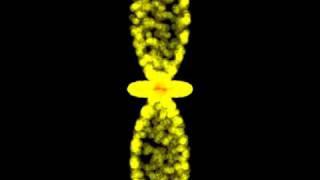
Is it going through nuclei ?
Ответить
Great animation. Would've been greater if you could express different phases in the motion with different colors. Anyway. Great! :)
Ответить
Electron is not a particle, you should not imagine it like a motionless volume
or as a point, is a moving shape, and its motion has rhythm and symmetry, like
all the existing things in the universe. Talking about probability of found an
electron is wrong; the electron is the shape and its motion. That shape moves rhythmically
due to the lack of equilibrium between the positive and negative energy. Going
from opposite shapes of maximum potential to minimum, again and again, but
unable to escape one from each other. But a subtle imbalance in that precarious
equilibrium can make the dance to come to an end. The electron comes off and that
beautiful shape collapse on itself moving away from the atom. Electron
is not a particle,, you should not imagine it like a motionles volumen or as a
point, is a moving shape, and its motion has rithm and simetry, like
all the existing things in the universe. Talking about probability of found an
electron is wrong, the electron is the shape and its motion. That shape
moves rithmicaly due to the lack of equilibrium between the postive and
negative energy. Going from oposite positions of máximum potential to mínimum,
again and again, but unable to escape one from each other.

This video is incredibly misleading...
Ответить
I've been watching this video since one year or so.. Can the motion be shown without the rotation of centre(nucleus) please?
Ответить
Awesome sauce!
Ответить
this is: wrong, misleading, WRONG.
So wrong.

Wow!!..thanks a lot...
Ответить
It's explain how a circular standing electron wave can gives an atomic orbital easily!!!
Ответить
This depiction is wrong.
Ответить
Rich pls give us an A
Ответить
I wonder if something like the primordial particle system can lead to electron behaviour. I'll reply with a video I made ( link)...
Ответить
How an electron can pass through nucleus?????? Its a wrong concept
Ответить
I was like waiting for all f orbitals
Ответить
This might be a dumb question, but I have always wondered this. The p,d, and f orbitals are always shown as crossing into the nucleus area, what prevents the electrons from entering the nucleus at this point because the EM force is so much stronger than the weak force. Also, how do these orbitals maintain the quantum energy levels when they get so mixed up in space.
Ответить
Just 1 lil mistake that elektrons dont touch proton nor other cloud
Ответить
TOTALLY WRONG
electrons don't move on trajectories well known, they just have probability densities, wich are highest inside the shape of the "electron clouds" as a fundamental thing on quantum mechanics, they just don't behave the way we're used to objects to behave, and can't measure the position and momentun at the same time, and also those have a limit, those shapes, which you programmed with python need numerical ansylisis and the schrödinger equation, which i am pretty much sure you know, so i think you just were playing with the program, but for the people who think this is how atoms behave, well, it is more complicated, and also the shapes of d orbitals are only possible with multiple electrons so it can't be just a matter of an electron orbiting, which means angular acceleration, which means electromagnetic waves. and we don't have that, we just have probability densities of where it's the most possible the electron to be, which is nearest to the proton on the hydrogen atom for example

All this in positive orientation, p orbital have 3 orientations and d,f have different orientations I think in this video all the shapes of these orbitals are shown in a specific orientation
Ответить
In this video electron really hit the nucleus , doesn't make any sense !
Ответить
I understood the electron was 2000 times smaller than the proton and the orbit or orbital was a long way away .
Ответить
In true scale--if that depicted nucleus were the actual size of the proton at the center of a hydrogen atom that you see here--the actual electron would be about 10 miles away! Atoms are mostly huge voids between objects just like our solar system.
Ответить
nobody:
electrons: 8

why electron of s not collide with electron of p orbital?
Ответить
Nope, these aren't wave functions. These animations show as if the electron has a trajectory, which it doesn't, but if we were to think of each frame of the electron as a measurement done, then yeah, it would make sense since it would give a probability distribution. But wave function? Not even close to it. The whole point of a wave function(more specifically, the wave function squared) is that the electron is in a superposition of all the probabilities, so giving specific trajectory to the electron doesn't make sense. It isn't moving through the orbital, it is, in a way, the orbital itself(at least according to quantum mechanics) since it is at all of the probabilities given with the orbital at once.
Ответить
Tank you
Ответить
I am not sure I understand. Is the electron jumping between orbitals?
Ответить
Wow
Ответить
me and the boyz watching DaAtomz spin
Ответить
Woow 👍🏻👍🏻👍🏻
Ответить
THIS is a WRONG model pf atom structure...please change your mind
Ответить
Legends watching after 11 years
Ответить
I think that this vdo is starting from n = 2 where standing wave forms by 2lambda , as the vdo starts electron is moving in 2s subshell later on on gaining energy it starts moving in 2p subshell then after I am not getting it . Whether I am thinking correct or wrong 🤔 help me
Ответить